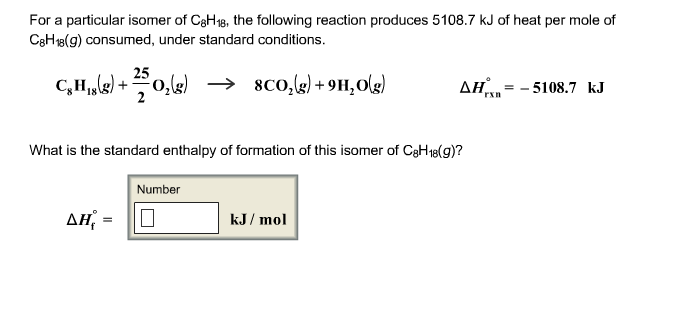
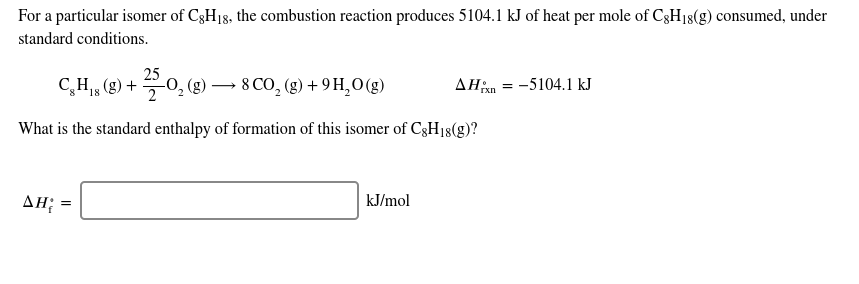
71+ pages for a particular isomer of c8h18 810kb. For a particular isomer of C8H18 the combustion reaction produces 51041 kJ of heat per mole of C8H18 g consumed under standard conditions. Solution for For a particular isomer of C8H18C8H18 the combustion reaction produces 51133 kJ51133 kJ of heat per mole of C8H18gC8H18g consumed under. Our experts in all academic subjects are available 247. Read also isomer and understand more manual guide in for a particular isomer of c8h18 3 question For a particular isomer of C8H18 the combustion reaction produces 51133 kJ of heat per mole of C8H18g consumed under standard conditions.
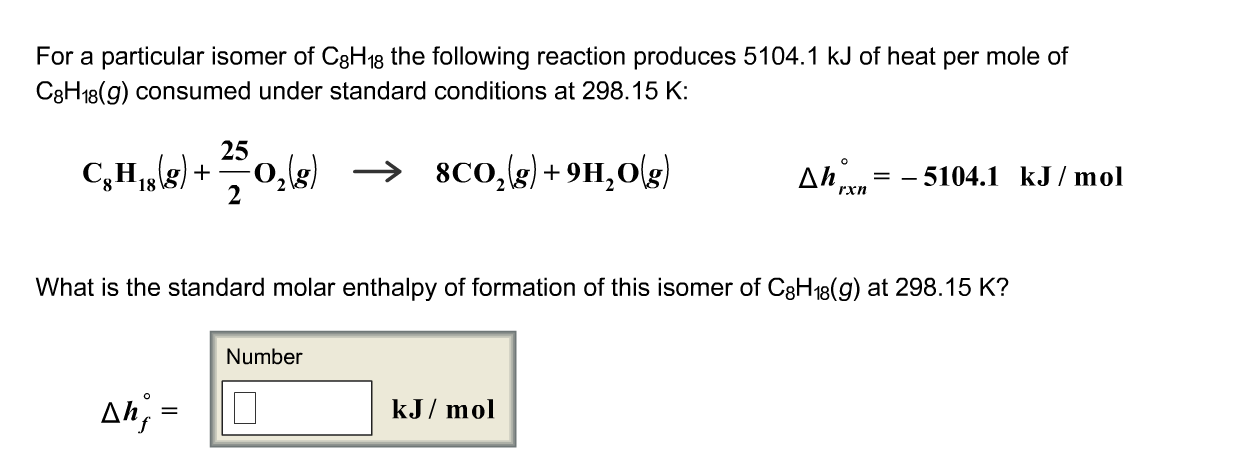
C8H18g252O2g 8CO2g9H2OgH rxn51133 kJmol What is the standard enthalpy of formation of this isomer of C8H18g. For a particular isomer of C8H18 the following reaction produces 50995 kJ of heat per mole of C8H18g consumed under standard conditionsC8H18g 252 O2g 8 CO2g 9 H2Og Hrxn -50995 kJMol What is the standard enthalpy of formation of this isomer of C 8H18g.
For A Particular Isomer Of C8h18 The Following Chegg
| Title: For A Particular Isomer Of C8h18 The Following Chegg |
| Format: eBook |
| Number of Pages: 169 pages For A Particular Isomer Of C8h18 |
| Publication Date: May 2019 |
| File Size: 6mb |
| Read For A Particular Isomer Of C8h18 The Following Chegg |
 |
For a particular isomer of C8H18 The following reaction produces 50937 KJ of heat per mole of C8H18 g consumed under standered conditions.

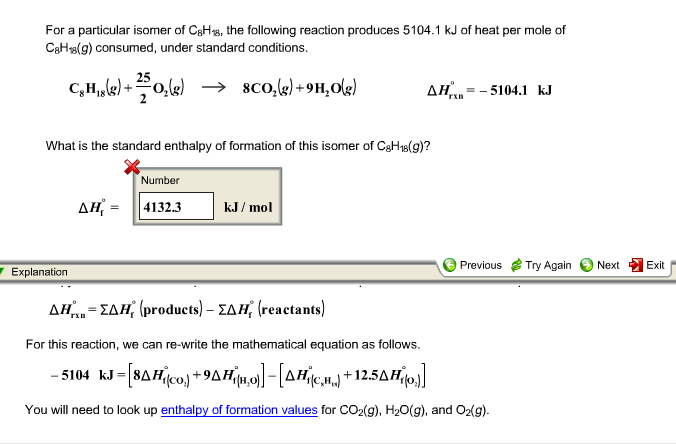
Correct answer - For a particular isomer of c8h18c8h18 the combustion reaction produces 50995 kj 50995 kj of heat per mole of c8h18gc8h18g consu. For a particular isomer of C8H18 The following reaction produces 50937 KJ of heat per mole of C8H18g consumed under standered conditions. We review their content and use your feedback to keep the. C8H18 g252O2 g8CO2 g9H2O grxn51087 kJmolC8H18 g252O2 g8CO2 g9H2O gHrxn51087 kJmol. 2 question For a particular isomer of c8h18c8h18 the combustion reaction produces 50995 kj 50995 kj of heat per mole of c8h18gc8h18g consumed under standard conditions. Experts are tested by Chegg as specialists in their subject area.
For A Particular Isomer Of C8h18 The Following Chegg
| Title: For A Particular Isomer Of C8h18 The Following Chegg |
| Format: PDF |
| Number of Pages: 297 pages For A Particular Isomer Of C8h18 |
| Publication Date: October 2017 |
| File Size: 1.3mb |
| Read For A Particular Isomer Of C8h18 The Following Chegg |
 |

For A Particular Isomer Of C8h18 The Bustion Reaction Pr Clutch Prep
| Title: For A Particular Isomer Of C8h18 The Bustion Reaction Pr Clutch Prep |
| Format: PDF |
| Number of Pages: 211 pages For A Particular Isomer Of C8h18 |
| Publication Date: September 2019 |
| File Size: 2.8mb |
| Read For A Particular Isomer Of C8h18 The Bustion Reaction Pr Clutch Prep |
 |
For A Particular Isomer Of C8h18 The Following Chegg
| Title: For A Particular Isomer Of C8h18 The Following Chegg |
| Format: eBook |
| Number of Pages: 246 pages For A Particular Isomer Of C8h18 |
| Publication Date: February 2017 |
| File Size: 2.6mb |
| Read For A Particular Isomer Of C8h18 The Following Chegg |
 |

For A Particular Isomer Of C8h18 The Following React
| Title: For A Particular Isomer Of C8h18 The Following React |
| Format: ePub Book |
| Number of Pages: 291 pages For A Particular Isomer Of C8h18 |
| Publication Date: January 2017 |
| File Size: 3.4mb |
| Read For A Particular Isomer Of C8h18 The Following React |
 |

For A Particular Isomer Of C8h18 The Foll Clutch Prep
| Title: For A Particular Isomer Of C8h18 The Foll Clutch Prep |
| Format: PDF |
| Number of Pages: 281 pages For A Particular Isomer Of C8h18 |
| Publication Date: November 2018 |
| File Size: 1.8mb |
| Read For A Particular Isomer Of C8h18 The Foll Clutch Prep |
 |
For A Particular Isomer Of C8h18 The Following Chegg
| Title: For A Particular Isomer Of C8h18 The Following Chegg |
| Format: ePub Book |
| Number of Pages: 257 pages For A Particular Isomer Of C8h18 |
| Publication Date: January 2019 |
| File Size: 1.7mb |
| Read For A Particular Isomer Of C8h18 The Following Chegg |
 |
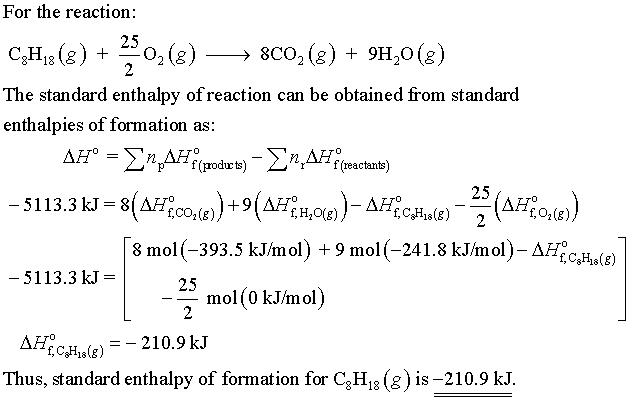
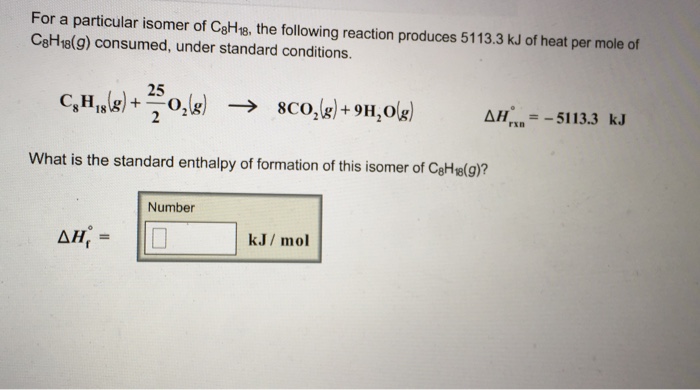
For A Particular Isomer Of C8h18 The Following Reaction Produces 5113 3 Kj Of Heat Per Mole Of C8h18 G Home Work Help Learn Cbse Forum
| Title: For A Particular Isomer Of C8h18 The Following Reaction Produces 5113 3 Kj Of Heat Per Mole Of C8h18 G Home Work Help Learn Cbse Forum |
| Format: PDF |
| Number of Pages: 332 pages For A Particular Isomer Of C8h18 |
| Publication Date: February 2017 |
| File Size: 1.7mb |
| Read For A Particular Isomer Of C8h18 The Following Reaction Produces 5113 3 Kj Of Heat Per Mole Of C8h18 G Home Work Help Learn Cbse Forum |
 |

For A Particular Isomer Of C8h18 The Bustion Reaction Pr Clutch Prep
| Title: For A Particular Isomer Of C8h18 The Bustion Reaction Pr Clutch Prep |
| Format: eBook |
| Number of Pages: 292 pages For A Particular Isomer Of C8h18 |
| Publication Date: June 2020 |
| File Size: 1.1mb |
| Read For A Particular Isomer Of C8h18 The Bustion Reaction Pr Clutch Prep |
 |
C8h18 Different Isomers
| Title: C8h18 Different Isomers |
| Format: eBook |
| Number of Pages: 164 pages For A Particular Isomer Of C8h18 |
| Publication Date: October 2021 |
| File Size: 2.1mb |
| Read C8h18 Different Isomers |
 |
For A Particular Isomer Of C8h18 The Bustion Chegg
| Title: For A Particular Isomer Of C8h18 The Bustion Chegg |
| Format: ePub Book |
| Number of Pages: 154 pages For A Particular Isomer Of C8h18 |
| Publication Date: September 2018 |
| File Size: 1.4mb |
| Read For A Particular Isomer Of C8h18 The Bustion Chegg |
 |
For A Particular Isomer Of C8h18 The Following Chegg
| Title: For A Particular Isomer Of C8h18 The Following Chegg |
| Format: PDF |
| Number of Pages: 328 pages For A Particular Isomer Of C8h18 |
| Publication Date: February 2019 |
| File Size: 5mb |
| Read For A Particular Isomer Of C8h18 The Following Chegg |
 |
Correct answer - For a particular isomer of c8h18c8h18 the combustion reaction produces 50995 kj 50995 kj of heat per mole of c8h18gc8h18g consu. What is the standard enthalpy of formation of this isomer. For a particular isomer of C8H18 the following reaction produces 51087 kJ of heat per mole of C8H18g consumed under standard conditions.
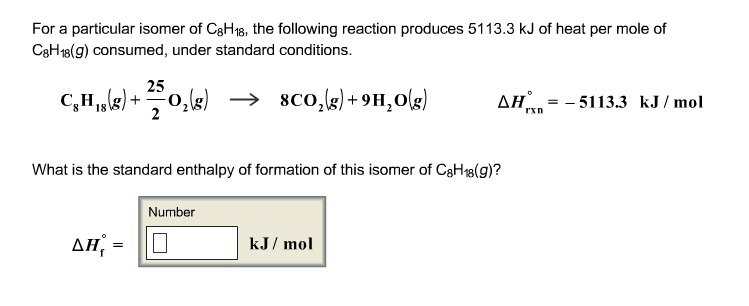
Here is all you need to know about for a particular isomer of c8h18 C8H18 g 252O2 g 8CO2 g 9H2O g Hrxn-51041 kJmol What is the standard enthalpy of formation of this isomer of C8H18 g. Dont hesitate to ask questions and start discussions whenever you need professional. Correct answer - For a particular isomer of C8H18 the combustion reaction produces 51133 kJ of heat per mole of C8H18g consumed under standard cond. For a particular isomer of c8h18 the following chegg for a particular isomer of c8h18 the following chegg for a particular isomer of c8h18 the following chegg for a particular isomer of c8h18 the foll clutch prep for a particular isomer of c8h18 the bustion reaction pr clutch prep c8h18 different isomers Who are the experts.
0 Comments